The aim of this review was to identify which treatment approach for pressure ulcers that currently are recommended by the National Institute for Clinical Excellence (NICE) guidelines and the latest Cochrane reviews on dressings, topical treatments and NPWT (a pump using vacuum to remove wound exudate) for pressure ulcers. The key statements and conclusions have been included below.
The review found:
1) Systemic antibiotics should not be used to treat a pressure ulcer, according to NICE.
2) Topical antiseptics or antimicrobials should not, according to NICE, routinely be used to treat a pressure ulcer. The Cochrane review found no data to indicate that any dressing type or antimicrobial were better than saline-soaked gauze. The Cochrane review even found itself unable to suggest what comparators to include in an RCT study.
3) NPWT (the pump) is not recommended by NICE for routine use on pressure ulcers, except to control the exudate. The Cochrane review could find no data to support its use in pressure ulcers.
4) NICE recommends considering moist wound care (covering the wound with an occlusive dressing). However, data indicate that the moist environment will exacerbate an infection (https://www.ncbi.nlm.nih.gov/pubmed/28532812). The Cochrane review, which is more recent, did indeed find no data to support this approach.
In conclusion, there are no data to support the use of any of the existing approaches for the treatment of pressure ulcers. This is in line with the recent study by Guest et al. (2018) that only 14% of infected pressure ulcers heal within the first 12 months. The use of antibiotics and antiseptics for other wound types have, in a similar manner, not demonstrated any clinical effects, which led both the FDA and NICE in 2016 to conclude that there is no effective treatment for infected wounds.
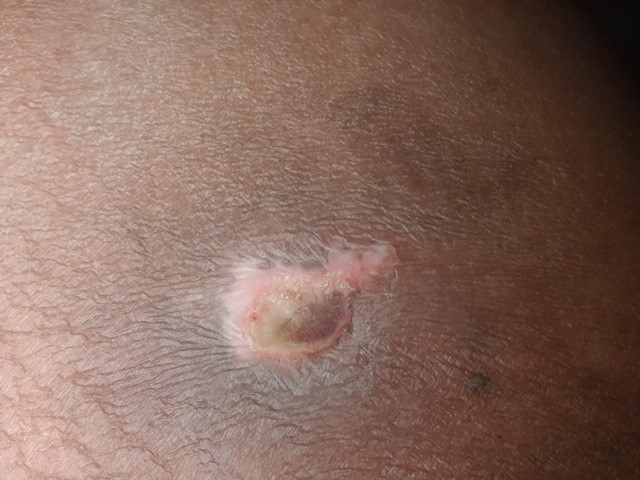
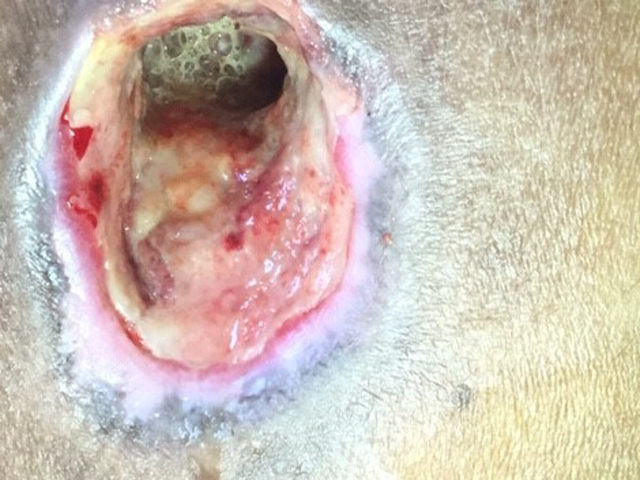
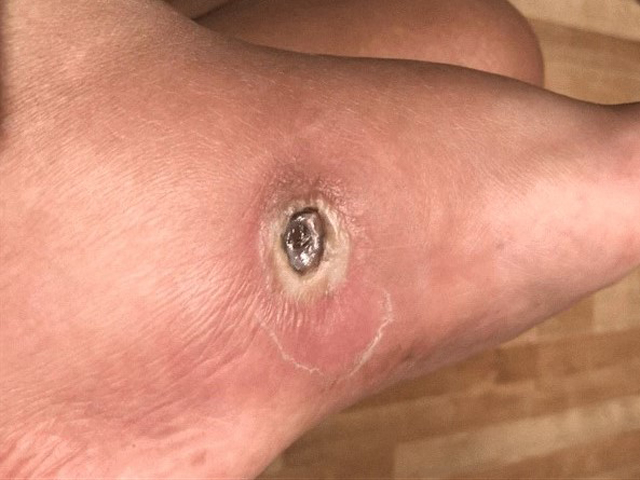
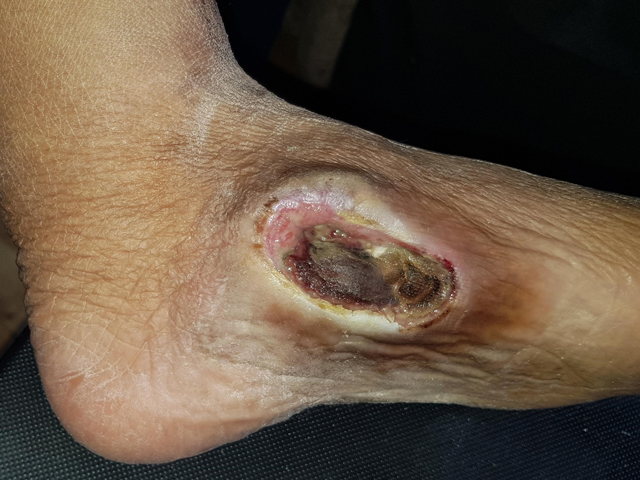
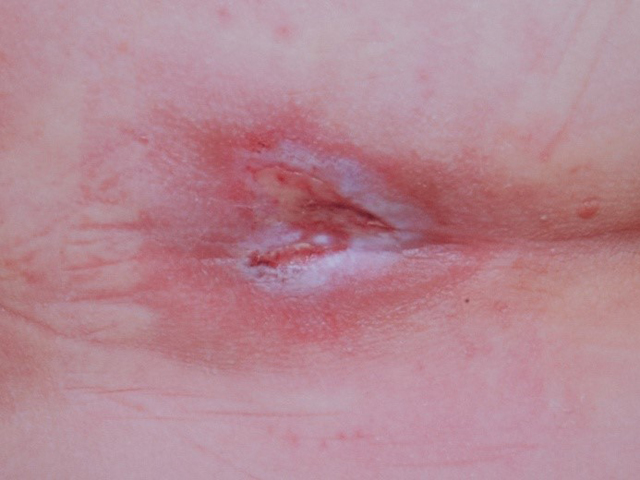
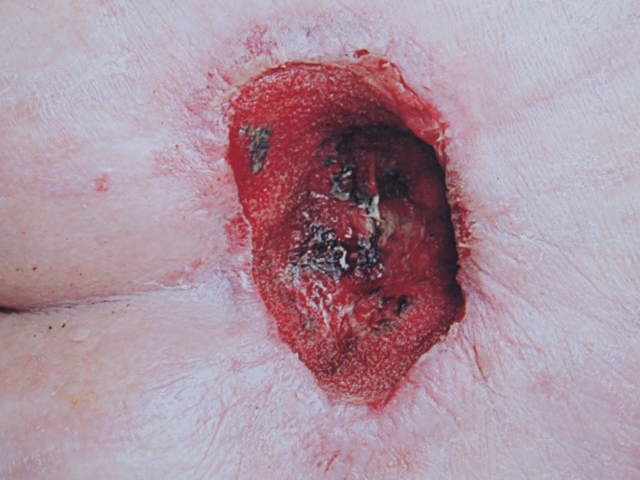
Examples of wounds deteriorating with standard-of-care treatment.
Top row: daily wound wash with Prontosan followed by application of silver dressings;
Middle row: iodine treatment twice weekly – the wound appeared in May and treatment with iodine was started in June and was has already by August deteriorating;
Bottom row: long array of dressings and NPWT were tried, but unsuccessfully.
Publications with excerpts:
NICE: Pressure ulcers: prevention and management - Clinical guideline.
Negative pressure wound therapy
1.4.13 Do not routinely offer adults negative pressure wound therapy to treat a pressure ulcer, unless it is necessary to reduce the number of dressing changes (for example, in a wound with a large amount of exudate).
Systemic antibiotics and antiseptics
1.4.18 After a skin assessment, offer systemic antibiotics to adults with a pressure ulcer if there are any of the following:
- clinical evidence of systemic sepsis (see also the NICE guideline on sepsis)
- spreading cellulitis
- underlying osteomyelitis
1.4.19 Discuss with a local hospital microbiology department which antibiotic to offer adults with infection to ensure that the chosen systemic antibiotic is effective
against local strains of infection.
1.4.20 Do not offer systemic antibiotics specifically to heal a pressure ulcer in adults.
1.4.21 Do not offer systemic antibiotics to adults based only on positive wound cultures without clinical evidence of infection.
Topical antimicrobials and antiseptics
1.4.22 Do not routinely use topical antiseptics or antimicrobials to treat a pressure ulcer in adults.
Dressings
1.4.23 Discuss with adults with a pressure ulcer and, if appropriate, their family or carers, what type of dressing should be used, taking into account:
- pain and tolerance
- position of the ulcer
- amount of exudate
- frequency of dressing change.
1.4.24 Consider using a dressing for adults that promotes a warm, moist wound healing environment to treat category 2, 3 and 4 pressure ulcers.
1.4.25 Do not offer gauze dressings to treat a pressure ulcer in adults.
Reference:
NICE: Pressure ulcers: prevention and management – Clinical guideline. Published: 23 April 2014. nice.org.uk/guidance/cg179
Westby et al. (2017) Dressings and topical agents for treating pressure ulcers
Authors conclusion:
A network meta‐analysis (NMA) of data from 39 studies (evaluating 21 dressings and topical agents for pressure ulcers) is sparse and the evidence is of low or very low certainty (due mainly to risk of bias and imprecision). Consequently we are unable to determine which dressings or topical agents are the most likely to heal pressure ulcers, and it is generally unclear whether the treatments examined are more effective than saline gauze.
More research is needed to determine whether particular dressings or topical agents improve the probability of healing of pressure ulcers. The NMA is uninformative regarding which interventions might best be included in a large trial, and it may be that research is directed towards prevention, leaving clinicians to decide which treatment to use on the basis of wound symptoms, clinical experience, patient preference and cost.
Reference:
Cochrane Systematic Review – Intervention Version published: 22 June 2017
Maggie J Westby, Jo C Dumville, Marta O Soares, Nikki Stubbs, Gill Norman
https://doi.org/10.1002/14651858.CD011947.pub2
Dumville et al. (2015) Negative pressure wound therapy for treating pressure ulcers
Main results:
The review contains four studies with a total of 149 participants. Two studies compared NPWT with dressings; one study compared NPWT with a series of gel treatments and one study compared NPWT with ‘moist wound healing’. One study had a 24-week follow-up period, and two had a six-week follow-up period, the follow-up time was unclear for one study. Three of the four included studies were deemed to be at a high risk of bias from one or more ‘Risk of bias’ domains and all evidence was deemed to be of very low quality. Only one study reported usable primary outcome data (complete wound healing), but this had only 12 participants and there were very few events (only one participant healed in the study). There was little other useful data available from the included studies on positive outcomes such as wound healing or negative outcomes such as adverse events.
Authors conclusion:
There is currently no rigorous RCT evidence available regarding the effects of NPWT compared with alternatives for the treatment of pressure ulcers. High uncertainty remains about the potential benefits or harms, or both, of using this treatment for pressure ulcer management.
Reference:
Cochrane Database Syst Rev. 2015 May 20;(5):CD011334. doi: 10.1002/14651858.CD011334.pub2.