Wounds, Infection and Antimicrobial Resistance
When a wound does not heal by itself, it is due to an infection. The severity of the infection depends, among other things, on the type of infection and on how old the wound is, i.e. how much the infection has been able to spread and invade surrounding tissues and structures.
Infections on the outside of the body, e.g. in wounds, are fundamentally different from infections within the body, where complete sterility must be kept. The definition of an infection within the body is, as it always has been, the presence of microorganisms. The conditions on the outside of the body, however, are quite different.
We are surrounded by and in constant contact with microorganisms. They are everywhere in our environment, on every surface we touch, and on us. They live on and in the skin. In fact, we cannot possibly keep our skin healthy without considering and looking after our over 1000 different species of microorganisms that live on us in an ecosystem called the skin microbiome. This microbiological community covers the entire skin surface, which in itself makes it difficult for disease-causing microbes to get a foothold. Our skin microbiome is constantly changing depending on our age, hormone status, food, climate, hygiene etc. and it is in constant communication with our immune system. The immune system, in turn, helps to maintain a good balance in the microbiome, ensuring a high degree of diversity and avoiding the dominance of one or a few species. Skin and wound infection are defined as an imbalance in the microbiome of such severity that the immune system has lost control of the area with the result that one or a few microbial species have become dominant and taken over control.
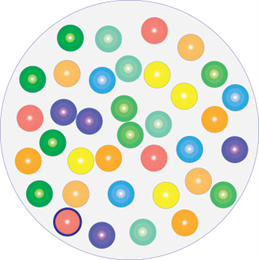
Healing wound.
Diverse microbiome

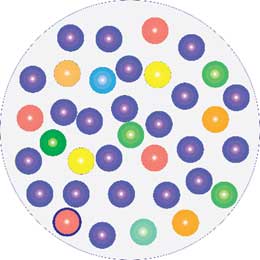
Infected wound.
One species has taken over control.
Infection is an unbalance in the microbiome
This relatively new understanding of what a wound infection really is, provides a logical explanation to why the FDA was right when they, as far back as 2016, following a thorough scientific review of the literature in the field, concluded, that antimicrobials do not work against wound infections. Antimicrobial agents such as antibiotics and antiseptics (e.g. silver, chlorhexidine, PHMB, etc.) kill the microbes without distinguishing which ones are too high or too low in numbers and whether their presence in the microbiome may actually be essential.
Antimicrobial resistance has become a genuine problem in recent years, and it is now known, that bacteria develop resistance to both antibiotics and antiseptics (disinfectants). Antiseptics can even create resistance to a type of antiseptics different to the one that caused the resistance, as well as to antibiotics (cross-resistance). Resistance development is targeted (not random), quick (few hours or days) and it is permanent (does not change back). In addition, the change is associated with a more aggressive behaviour (virulence) in the bacteria. This knowledge has only been established within the last few years and, in practical terms, it means that the problem of antimicrobial resistance is not limited to the fact that our antimicrobials do not work, but also that using antimicrobials in the wrong places, such as for example in wounds, accelerates the development and spread of resistance, as well as makes the individual infection more dangerous.
Today, as we can all be expected to carry at least one resistant bacterium in our skin microbiome, the use of antimicrobial agents will not eradicate all the microbes in the wound. The resistant type will not be affected but will remain in the wound, whereas the non-resistant types will be killed. Put into practice, this means that the treatment specifically favours and selects for the resistant bacterial type providing it with ideal conditions to multiply, occupy and control / infect the entire wound. The result is a wound full of resistant, highly aggressive (virulent) bacteria. The use of antimicrobials in wounds can therefore no longer simply be regarded as something harmless that just does not work. On the contrary, it poses a danger as it aggravates the wounds and reinforces and spreads resistance to the patient and to those with whom the patient comes into contact.

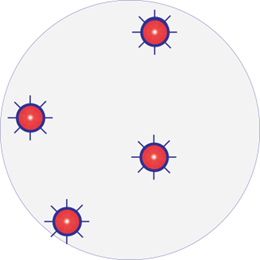
Infected wound with resistant bacteria
One species dominating. Resistant strain: Red with border
After treatment with antimicrobials
Resistant strains survive and become more aggressive
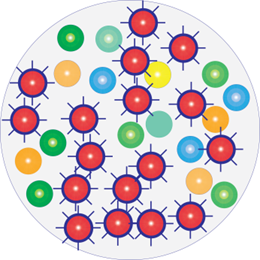
A few hours following antimicrobial treatment
Infection has worsened and become more dangerous
With Resistant Bacteria: Washing, dressing or treating wounds with products that kill bacteria (antimicrobials) makes the infection worse and more dangerous.
The only way to control a wound infection is therefore to restore the balance between the microbes in the skin microbiome. Since the skin microbiome is unique to the individual and can be composed of many different species and is constantly changing, it is virtually impossible for us to know what the ideal composition is at any point in time. The individual’s immune system, however, knows this, because it is constantly in contact with the microbes and maintains the balance.
A bacterium that tries to dominate and thus create infection will typically secrete toxins and enzymes to kill the immune cells and other types of bacteria, and will hide within the biofilm, which is a house it previously built and shared with the other microbes. It is difficult for the immune cells to penetrate biofilms, and perhaps it becomes even more difficult for them when certain selected highly aggressive bacteria take over the sole responsibility for the construction of the biofilm house and possibly turn it into a fortress.
Amicapsil (micropore particle technology or MPPT) helps the body’s immune system as much as possible to regain control of the area. It removes the toxins so that the immune cells do not die and it helps make holes in the biofilm so that the immune cells can gain access. Once inside the biofilm, the immune cells can selectively tidy up and restore the diversity and balance in the composition of microbes. Doing this, removes the infection and brings the wound back on the natural path to healing. Indeed, it is also the immune system that controls all the processes associated with the reconstruction of lost tissue (muscle, connective tissue, vessels, skin, etc.) throughout the process of closing the wound.
Amicapsil has been approved in the UK and throughout the EU since 2016 for use by professionals and 2017 for use by individuals and it has been extensively tested. It has no side effects, and it neither contributes to new antimicrobial resistance; spread resistance; nor reduce biodiversity. By preventing antimicrobials pervading nature, it makes a valid contribution to the net-zero target.














